Covid-19
Journal of the American Heart Association: published
on Jun 2022
COVID‐19 is an infectious illness,
with an increased risk of thromboembolism. The overall rate of venous
thromboembolism and arterial thromboembolism among patients with COVID‐19 is
21% and 2%, respectively.
Therefore, this complication
represents an important therapeut...
Clinical Infectious Diseases: An Official Publication of the Infectious
Diseases Society of America: Published on June 2022
COVID rebound has been reported to
occur 2 to 8 days after initial recovery and is characterized by a recurrence
of COVID-19 symptoms or a positive viral test after having tested negative. The
CDC recommends that he...
European Heart Journal:
Management of
cardiometabolic risk factors should become a priority for physicians. The
long-term impact of COVID-19 on cardiovascular (CV) health and mortality is
also emerging as a major global concern.
Non-pharmacological
supportive approaches:
The management of long COVID tends to be
largely supportive....
Nature Medicine
Post-acute
sequelae of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)—can
involve the pulmonary and several extrapulmonary organs, including the
cardiovascular system. A few studies have investigated cardiovascular outcomes
in the post-acute phase of the COVID-19; however, most were limited to
hospitalized...
Children who are infected with
COVID-19 are at a higher risk of developing diabetes, according to a new study
by the Centers for Disease Control and Prevention (CDC). Increased incidence of
diabetes seen among patients aged younger than 18 years after acute COVID-19
infection versus those without COVID-19.
Key Summary
What is already...
Pre-Clinical Phase
Collects data to support feasibility and safety
Involves iterative animals testing such as mice or
monkeys to see if it produces an immune response.
Evaluates toxic and pharmacological effects
Normally occurs before human testing can begin
Sponsored content...
The rapidly escalating coronavirus
disease 2019 (COVID-19) pandemic has focused attention on the diagnosis and
treatment of patients with acute respiratory infection in an unprecedented
manner. Although most of the lung injury patients have is believed to be caused
by the virus, concern over bacterial co-infection also informs current
treatmen...
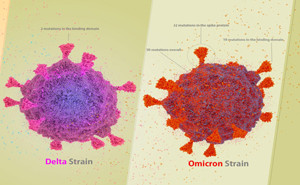
Comparing COVID-19 variants (Delta vs. Omicron) RemoveAvailable Brand
COVID-19 Update:In light of the most recent
information and data available, today, the FDA revised the authorizations for
two monoclonal antibody treatments – bamlanivimab
and etesevimab (administered together) and casirivimab and imdevimab – to limit their use to only when the
patient is likely to have been infected with or exposed to a varian...
The FDA announced it had amended
the emergency use authorizations for both the Moderna and Pfizer-BioNTech
vaccines allowing for a single booster dose for all individuals 18 years of age
and older after completion of primary vaccination with any of the approved COVID-19
vaccines.The CDC also recommends that
adults should get a booster at least...
The U.S. Food and Drug
Administration authorized Pfizer’s Paxlovid (nirmatrelvir tablets and ritonavir
tablets, co-packaged for oral use) for emergency use for the treatment of
mild-to-moderate coronavirus disease in adults and children 12 years and older weighing
at least 40 kilograms with positive results of direct SARS-CoV-2 testing, and who...
The U.S. Centers for Disease
Control and Prevention announced that the recommended isolation time for
people with COVID-19 is now reduced from
10 to 5 days for those who are asymptomatic.
The agency said that people who test
positive should isolate for 5 (five) days, and if they are asymptomatic, or
their symptoms are resolving (without fe...