Allergy-immune-system-drugs
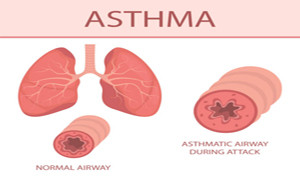
Asthma, a highly prevalent chronic
inflammatory disease of the airways, is characterized by a variable degree of
airflow obstruction, edema, and bronchial hyper responsiveness caused by
chronic exposition to allergens or associated to other diseases such as gastro
esophageal reflux. The complex genetic actions and
environmental triggers intric...
JAMA Network: Published on June, 2022Montelukast, the most widely used
leukotriene-modifying agent (LTMA), is a selective leukotriene receptor
antagonist and is currently indicated for prophylactic and chronic treatment of
asthma, relief of symptoms of allergic rhinitis, and acute prevention of
exercise-induced bronchoconstriction.This propensi...
Efficacy of Montelukast in preventing seasonal recurrence of vernal keratoconjunctivitis in Children
TAKE-HOME MESSAGEVernal keratoconjunctivitis is a
chronic, seasonally exacerbated, allergic inflammation of the eye.This prospective study evaluated
the efficacy and safety of montelukast in conjunction with typical topical
therapies for treating pediatric vernal keratoconjunctivitis (VKC). Patients
were evaluated for subjective improvement in...
PubMed Central: Published: July, 2021
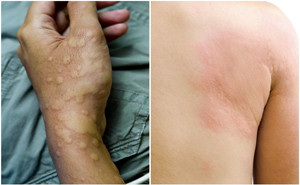
Urticaria is a frequent, mast cell–driven disease that
presents with wheals, angioedema, or both. The lifetime prevalence for acute
urticaria is approximately 20%.
Chronic idiopathic urticaria (CIU) is a common skin condition
characterized by the appearance of recurrent wheals and/or angioede...
The U.S. Food and Drug
Administration revised the emergency use authorization (EUA) of bamlanivimab
and etesevimab monoclonal antibodies (previously authorized for pediatric
patients 12 years of age and older weighing at least 40 kilograms, or about 88
pounds), to additionally authorize bamlanivimab and etesivimab administered
together for the...
Sotrovimab has been approved for patients with mild-to-moderate
COVID-19 who are at high risk of developing severe disease.The Medicines and Healthcare
products Regulatory Agency (MHRA) has approved the monoclonal antibody
treatment Sotrovimab (Xevudy; GSK and Vir Biotechnology) for people with
mild-to-moderate COVID-19 and at least one risk fa...
The U.S. Food and Drug Administration
authorizes bamlanivimab and
etesevimab, administered together, to include emergency use as post-exposure
prophylaxis (prevention) for COVID-19 in adults and pediatric patients (12
years of age and older weighing at least 40 kg) who are at high risk for
progression to severe COVID-19, including hospitalizat...
Gamaleya Research Institute of Russia on August 11 became the first
country in the world to have registered a vaccine against the coronavirus - Sputnik V which is Adeno-based Non-replicating
viral vector. It is a combination of two adenoviruses, Ad5 and Ad26, both
engineered with a coronavirus gene.
“The vaccination of first...
The U.S. Food and Drug
Administration issued an emergency use authorization (EUA) for
bamlanivimab and etesevimab administered together for the treatment of mild to
moderate COVID-19.
In a clinical trial of patients
with COVID-19 at high risk for disease progression, a single intravenous
infusion of bamlanivimab and etesevimab administe...
Probiotics are live
microorganisms that confer health benefits when consumed in adequate amounts,
including enhanced immune activity and the clearance of respiratory tract
infections. (FAO and WHO, UN).
Probiotics could be used as an
adjunctive treatment against COVID-19.Role of probiotics to
combat COVID-19It is evident that probiotics ca...
Answers of your Queries about Covid Vaccine.Questions regarding Vaccine administration and safety.
COVID-19 Vaccine FAQs from
Patient’s to Healthcare Professionals.Questions regarding Vaccine Indications.