What Doctor's need to know about Oxford's Covishield Corona Vaccine (P1)
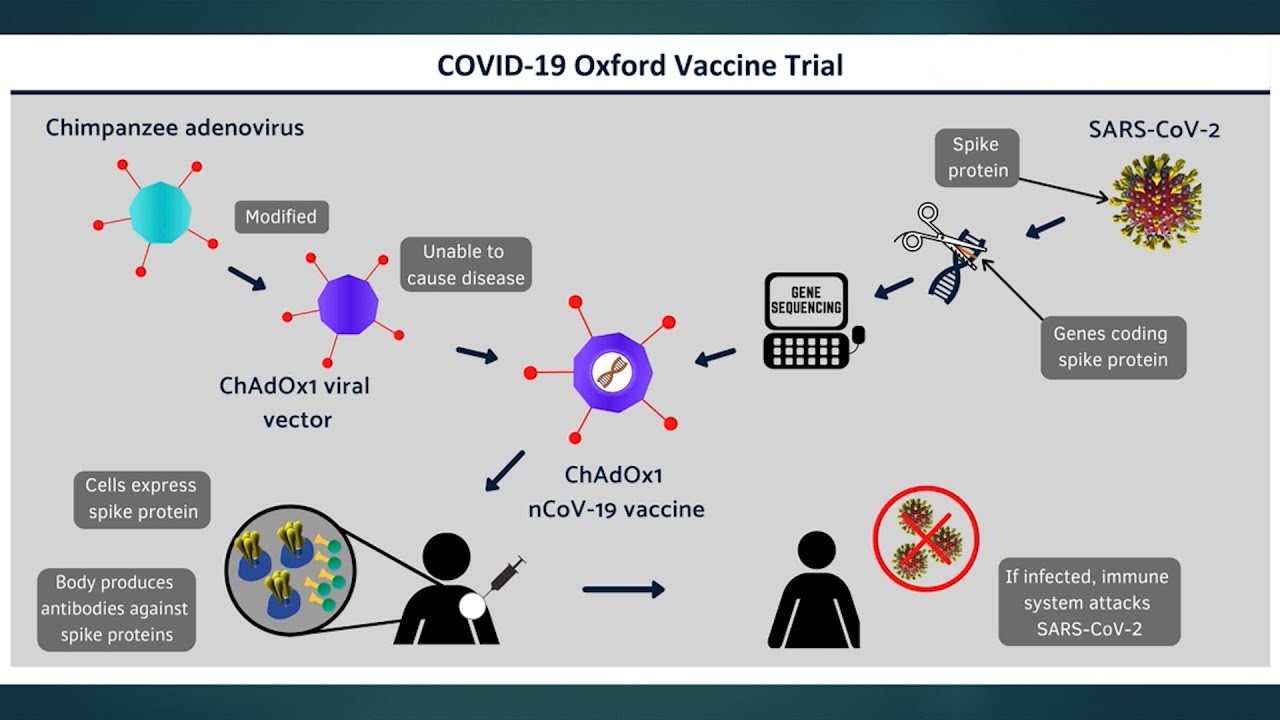
The ChAdOx1 nCoV-19 vaccine was
developed by the University of Oxford and AstraZeneca and manufactured in Serum
Institute of India.
The vaccine works by delivering
the genetic code of the SARS-CoV-2 spike protein to the body’s cells, similarly
to the BNT162b2 vaccine. Once inside the body, the spike protein is produced,
causing the immune system to recognize it and initiate an immune response. This
means that if the body later encounters the spike protein of the coronavirus,
the immune system will recognize it and destroy it before causing infection.
Doctors Liked to Read More
Safety and efficacy of the ChAdOx1
nCoV-19 vaccine (AZD1222) against SARS-CoV-2
According to the trial report,
the AstraZeneca-Oxford vaccine is about 70% effective on average. However, this
average was calculated after a 62% effective rate was observed in people who
received the full vaccine dose compared with 90% effective in those who
received the half dose.
The data also suggest it can
reduce spread of Covid, as well as protect against illness and death.
The most frequently reported adverse reactions were -
- injection site tenderness (>60%);
- injection site pain, headache, fatigue (>50%);
- myalgia, malaise (>40%);
- pyrexia, chills (>30%);
- arthralgia, nausea (>20%).
The majority of adverse reactions were mild to moderate in severity and usually resolved within a few days of vaccination. By day 7 the incidence of subjects with at least one local or systemic reaction was 4% and 13% respectively. When compared with the first dose, adverse reactions reported after the second dose were milder and reported less frequently.
Adverse reactions were generally milder and reported less frequently in older adults (≥65 years old).
If required, analgesic and/or anti-pyretic medicinal products (e.g. paracetamol-containing products) may be used to provide symptomatic relief from post-vaccination adverse reactions.
The first full results from the interim analysis of the Oxford-AstraZeneca vaccine have shown that only three out of 24,000 participants had an adverse reaction to the vaccine. No cases of hospitalisation or death were reported among those who were given the vaccine. This data comes from the world's first peer-reviewed scientific publication of Phase 3 trial results of any COVID-19 vaccine.
Pregnancy and breastfeeding
None of the trials conducted on the currently available vaccines have included pregnant or breastfeeding women. There have, however, been people who became pregnant during the trials, and they and their babies are being monitored closely.
At the moment, there isn’t any sign that these vaccines cause harm to pregnant women or the unborn baby. However, there isn’t yet enough data to say that they should be given in pregnancy. It is expected that there will be more trials and data on this in future.
For cases where a pregnant woman is at high risk of exposure to the virus or they are at very high risk of having serious complications from COVID-19, they should discuss the risks and benefits of being vaccinated with their clinician.
The JCVI recommended that women who are breastfeeding may be offered both the Pfizer-BioNTech and Oxford-AstraZeneca vaccines. There is no known risk associated with receiving non-live vaccines whilst breastfeeding.
Children
Children and young people have a very low risk of becoming seriously ill from COVID-19 disease compared to adults. At the moment, children under the age of 18 have not been included in the vaccination recommendations for Oxford vaccine. Some children with complex medical conditions may be advised to receive a COVID-19 vaccine, but this assessment will be made on an individual basis. Clinical trials are being planned or already underway to provide evidence for use in this age group.
Allergies
There were no serious allergic reactions during any of the trials of the vaccines. None of these trials included participants who were known to have severe allergies.
The vaccine should also not be given to anyone who has had a previous ‘systemic allergic reaction (including immediate onset anaphylaxis)’ to ‘any component of the vaccine’, or who had such a reaction to their first dose of the vaccine.
The Oxford-AstraZeneca vaccines should not be given to those who have had a previous severe allergic reaction to:
- A previous dose of the same COVID-19 vaccine
- An ingredient in of the COVID-19 vaccine
The Oxford-AstraZeneca vaccine does not contain PEG, so those with allergies to this ingredient can receive the alternative vaccine.
Pregnancy
There is a limited experience with the use of COVID-19 Vaccine AstraZeneca in pregnant women. There are no specific safety concerns with the vaccines - but they were not tested on pregnant women.
Preliminary animal studies do not indicate direct or indirect harmful effects with respect to pregnancy, embryofetal development, parturition or post-natal development; definitive animal studies have not been completed yet. The full relevance of animal studies to human risk with vaccines for COVID-19 remains to be established.
Administration of COVID-19 Vaccine AstraZeneca in pregnancy should only be considered when the potential benefits outweigh any potential risks for the mother and fetus.
Breastfeeding
It is unknown whether COVID-19 Vaccine AstraZeneca is excreted in human milk.
Women who are breastfeeding can be given Covid vaccine.
Updated advice from the JCVI following authorization of the Oxford/AstraZeneca COVID-19 vaccine has said pregnant & breastfeeding women should be considered for vaccination if they are at high risk of exposure to COVID-19 or at risk of serious complications.
The Joint Committee on Vaccination and Immunization (JCVI)has said that while there were no known risks associated with pregnant or breastfeeding women receiving non-live vaccines there was 'insufficient evidence to recommend routine use of COVID-19 vaccines during pregnancy'.
Pregnant Women
However, it said it was now advising that vaccination should be considered for pregnant women where 'the risk of exposure to SARS-CoV2 infection is high and cannot be avoided, or where the woman has underlying conditions that put them at very high risk of serious complications of COVID-19.'
However, the JCVI now advises that if a pregnant woman meets the definition of being clinically extremely vulnerable, then she should discuss the options of COVID-19 vaccination with her obstetrician and/or doctor. This is because their underlying condition may put them at very high risk of experiencing serious complications of COVID-19. The most likely relevant groups of pregnant women are:
- Solid organ transplant recipients
- Those with severe respiratory conditions including cystic fibrosis and severe asthma
- Those who have homozygous sickle cell disease
- Those receiving immunosuppression therapies sufficient to significantly increase risk of infection
- Those receiving dialysis or with chronic kidney disease (stage 5)
- Those with significant congenital or acquired heart disease
Additionally, pregnant women who are frontline health or social care workers, including carers in a residential home, can also discuss the option of vaccination. This is because the risk of exposure to COVID-19 may be higher, even if they have a lower risk of experiencing complications if they are otherwise well.
Breastfeeding Women
The JCVI also now advises that there is no known risk in giving these vaccines to breastfeeding women. Breastfeeding women should therefore be offered vaccination if they are otherwise eligible, for example if they are a frontline health or social care worker, including a carer in a residential home. Women should be advised that there is lack of safety data for these specific vaccinations in breastfeeding.
Yes. The guidance has also been updated to say that women do not need to avoid pregnancy after vaccination. People who want to get pregnant in the future may receive the COVID-19 vaccine.
Based on current knowledge, experts believe that COVID-19 vaccines are unlikely to pose a risk to a person trying to become pregnant in the short or long term.
Previous guidance had suggested women should try to avoid conceiving for three months after receiving the vaccine.
Yes. If eligible for a flu vaccine, he/she should get it as soon as possible, particularly if they will also be in a high-risk priority group for a Covid vaccination.
The Joint Committee on Vaccination and Immunization (JCVI) recommended leaving at least seven days between the vaccines.
No. GPs do not need to observe patients for 15 minutes after administering the Oxford/AstraZeneca Covid vaccine.
The MHRA said this comes as no reports of anaphylactic adverse reactions have so far been associated with the Oxford vaccine.
Patients vaccinated with the Pfizer/BioNTech vaccine must still be kept for observation for 15 minutes after receiving their jab.
In an update to the standard operating procedures for vaccination, NHS England said: ‘For the Oxford/AstraZeneca there is not a requirement for 15 minutes observation unless this is indicated after clinical assessment.’
‘The fifteen-minute observation period time was introduced for the Pfizer vaccine guidance as a precaution following early reports of anaphylaxis following vaccination. ‘Such reports have so far not been observed with the AstraZeneca vaccine.
However, as with all injectable vaccines, appropriate medical treatment and supervision should always be readily available in case of an anaphylactic event following the administration of the vaccine.’
“The information in this article is accurate as of publication time. However, as the situation surrounding COVID-19 continues to evolve, it's possible that some data may have changed since publication.”
https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(20)32661-1/fulltext
https://www.gponline.com/updated-jcvi-advice-says-at-risk-pregnant-women-receive-covid-19-vaccine/article/1703502
https://www.rcog.org.uk/en/news/updated-advice-on-covid-19-vaccination-in-pregnancy-and-women-who-are-breastfeeding/
https://www.rcog.org.uk/en/guidelines-research-services/guidelines/coronavirus-pregnancy/covid-19-virus-infection-and-pregnancy/
https://www.bmj.com/content/372/bmj.n64
https://www.cdc.gov/coronavirus/2019-ncov/vaccines/facts.html
https://vk.ovg.ox.ac.uk/vk/covid-19-vaccines
https://www.bbc.com/news/uk-wales-55484408
https://www.telegraph.co.uk/global-health/0/covid-19-vaccine-news-updates-latest-oxford-astrazeneca-moderna-pfizer/
https://www.verywellhealth.com/astrazeneca-oxford-covid-19-vaccine-5093148#who-can-get-the-astrazeneca-vaccine
https://www.pulsetoday.co.uk/news/clinical-areas/allergy/no-requirement-to-observe-patients-for-15-minutes-after-oxfordastrazeneca-covid-vaccination/
https://www.gov.uk/government/publications/regulatory-approval-of-covid-19-vaccine-astrazeneca/information-for-healthcare-professionals-on-covid-19-vaccine-astrazeneca
https://www.ox.ac.uk/news/2020-11-19-oxford-coronavirus-vaccine-produces-strong-immune-response-older-adults
https://www.sciencefocus.com/news/covid-19-oxford-vaccine-produces-robust-response-in-older-adults/
https://www.health.com/condition/infectious-diseases/coronavirus/if-you-already-had-covid-do-you-need-vaccine
https://www.hopkinsmedicine.org/health/conditions-and-diseases/coronavirus/covid-19-vaccine-what-you-need-to-know




Comments
You must login to write comment