Featured
JAMA: The Journal of the American Medical Association: Published on
November, 2022Despite advances in treatment of
COVID-19, additional therapies are needed, particularly in the outpatient
setting. Novel oral antivirals have been authorized for high-risk individuals. Numerous
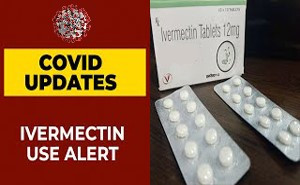
repurposed drugs have been investigated for COVID-19.Ivermectin, an a...
NICE guidelines: The holistic assessment, investigation, and management approach suggested by NICE are outlined here.Pulmonary Symptoms ManagementInvestigations:Pulmonary symptoms are common during long covid. NICE recommends that breathlessness may be investigated using an exercise tolerance test suited to the person’s ability, for example the one...
The New England Journal of MedicineMolnupiravir is an orally
administered form of a potent ribonucleoside analog that inhibits the
replication of SARS-CoV-2. For nonhospitalized, unvaccinated adults with
mild-to-moderate COVID-19 and at least one risk factor for severe disease,
molnupiravir reduces the risk for hospitalization or death, accordi...
The U.S. Food and Drug
Administration approved the emergency use of the molnupiravir pill for the
treatment of mild-to-moderate coronavirus disease 2019 in nonhospitalized adult patients with positive
results of direct SARS-CoV-2 viral testing who are at high risk for progressing
to severe COVID-19, including hospitalization or death.Does molnu...
Omicron driving spike in new Covid-19 cases in Bangladesh
recently and expected to rise in coming days according to health officials. The Covid-19 positivity rate in Bangladesh has reached the
highest-ever 33.37% in last week. The health authorities recorded 15,440 new infections after
testing 46,268 samples across the country. The latest figure...
The National Institute for Health and Care Excellence (NICE) GuidanceManaging cough Recommendations· Encourage patient
with cough to avoid lying on their backs, if possible, because this may make
coughing less effective.· Be aware
that older people or those with comorbidities, frailty, impaired immunity or a
reduced ability to cough...
Nirmatrelvir and Ritonavir is the
first oral agent authorized by FDA (Emergency Use Authorization) for treatment
of mild-to-moderate coronavirus disease in adults and
pediatric patients.
In a randomized, double-blind,
placebo-controlled clinical trial, Nirmatrelvir and Ritonavir significantly
reduced the proportion of people with COVI...
The U.S. Food and Drug
Administration (USFDA) issued an emergency use authorization (EUA) for a new
monoclonal antibody for the treatment of COVID-19 that retains activity against
the omicron variant.
The EUA for Bebtelovimab is for the treatment of mild to moderate COVID-19 in
adults and pediatric patients (12 years of age and older we...
The COVID-19 Treatment Guidelines:
On December 22 and 23, 2021, the
Food and Drug Administration (FDA) issued Emergency Use Authorizations (EUAs)
that allow 2 new oral antiviral agents to be used in this patient population:
ritonavir-boosted nirmatrelvir and molnupiravir.
Ritonavir-Boosted Nirmatrelvir
Nirmatrelvir is an orally bi...
The New England Journal of Medicine
TAKE-HOME MESSAGE
This retrospective study evaluated
the effectiveness of the Pfizer (BNT162b2) COVID-19 vaccine in preventing
reinfection in patients who had recovered from COVID-19.
A total of 149,039 patients who had
recovered from COVID-19 were included, and, of these, 56% were subsequently...
The use of empiric antibiotic therapy
appears to prevent neither deterioration nor death among patients with COVID-19
pneumonia, as shown in this study.
Take Home Message
In a group of patients with
COVID-19 pneumonia, antibiotics were commonly started in those who were
severely ill. Patients who did vs did not receive antibiotics mor...
The New England Journal of Medicine
Infection
with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and illness
with the associated coronavirus disease 2019 (Covid-19) continue to threaten
global health.
Persons
with particular characteristics such as older age, current smoking, or
underlying clinical conditions such as...