Featured
A study by the University of
Oxford, published in the journal Cell, demonstrates that currently
available vaccines, including AstraZeneca’s COVID-19 vaccine, will provide
protection against the Delta (B.1.617.2) and Kappa (B1.617.1) variants;
formerly the ‘Indian’ variants.
The study, investigated the
ability of antibodies in the blood of...
Oxford researchers say having
AstraZeneca then Pfizer vaccine is almost as potent as two shots of Pfizer.Scientists in Oxford looked at
the impact of a mix-and-match approach to vaccinations where people were given
either the standard two shots of Oxford/AstraZeneca or Pfizer/BioNTech
vaccine, or a combination of the two. Mixed schedules involv...
The AstraZeneca-Oxford vaccine is
a recombinant adenoviral vector vaccine. Recombinant vaccines use a small piece
of genetic material from a pathogen, like SARS-CoV-2, to trigger an immune
response. A specific piece of the virus can be targeted, and recombinant
vaccines are generally safe to use in a large population of people—even those
with...
The ChAdOx1 nCoV-19 vaccine was
developed by the University of Oxford and AstraZeneca and manufactured in Serum
Institute of India.
The vaccine works by delivering
the genetic code of the SARS-CoV-2 spike protein to the body’s cells, similarly
to the BNT162b2 vaccine. Once inside the body, the spike protein is produced,
causing the immune...
The Oxford University
AstraZeneca coronavirus vaccine has been approved for use in the UK.
The Oxford University/AstraZeneca vaccine has been approved
by the UK medicines regulator, opening up the possibility of rapidly scaling up
vaccination against Covid-19 within days.
The Medicines and Healthcare products Regulatory Authority
(MHRA) has approved the 2 doses vaccine for use in the UK. Because it needs
only norma...
Pharmaceutical company AstraZeneca and
Oxford University have resumed clinical trials of their COVID-19 vaccine
candidate in the United Kingdom after a brief global pause in testing.
AstraZeneca
put a hold on its COVID-19 clinical trials worldwide this week while it
investigated an adverse reaction in...
Oxford
researchers halt vaccine trial while adverse reaction is investigated
One
of the leading covid-19 vaccine candidate trials has been voluntarily paused as
part of a standard review process triggered by a “single event of an
unexplained illness that occurred in the UK phase III trial.”
The...
Beximco Pharmaceuticals, one of
the leading drug makers of the country, announced today it will invest with
Serum Institute of India (SII) to ensure Bangladesh receives Covid-19 vaccine
on a priority basis, once approved by the regulator.
In a press statement, Beximco
Pharmaceuticals said it will make a financial contribution...
Background: The Oxford Vaccine Group at the University of Oxford
are developing a new vaccine candidate for COVID-19, a chimpanzee adenovirus
vaccine vector called AZD1222 (previously ChAdOx1). The team has previously
developed a MERS vaccine.
In India, the candidate is being
jointly developed by the Serum Institute of India...
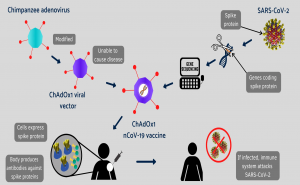
A diagram showing how the Oxford COVID-19 vaccine works. A chimpanzee
adenovirus is used in the ChAdOx1 viral vector, engineered to match the
SARS-CoV-2 spike protein.
The University of Oxford vaccine
is delivered via a chimpanzee virus, called the vaccine vector. The vector
contains the genetic code of t...
A vaccine in development by the
British-Swedish company AstraZeneca
and the University of Oxford is
based on a chimpanzee adenovirus called ChAdOx1.
Their Phase 1/2 trial revealed that the vaccine was safe, causing no severe
side effects. It raised antibodies against the coronavirus as well as other
immune defenses....