Coronavirus-vaccine-updates
Background: Biotech Company Novavax announced in March that it has produced a stable, prefusion protein nanoparticle vaccine candidate for COVID-19. A Phase 1/2 trial evaluating NVX-CoV2373 began on 25 May. Study Design: A randomized, observer-blinded, placebo-controlled trial of 130 healthy participants 18 to 59 years of age at two s...
NVX‑CoV2373 is a vaccine candidate engineered from the genetic sequence of SARS‑CoV‑2, the virus that causes COVID-19 disease. NVX‑CoV2373 is a stable, prefusion protein made using Novavax’ proprietary nanoparticle technology and includes Novavax’ proprietary Matrix‑M™ adjuvant.NVX‑CoV2373 was created using Novavax’ recombinant nanop...
Maryland-based Novavax makes vaccines by sticking proteins onto microscopic particles. Engineered from the genetic sequence of COVID-19, we used our recombinant nanoparticle technology to generate antigen derived from the coronavirus spike protein. The company launched trials for a Covid-19 vaccine in May.Phase 1 & Phase 2 TrialIn t...
Background: mRNA-1273 was developed by Moderna based on prior
studies of related coronaviruses such as those that cause severe acute
respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS). A
Phase 1 trial (NCT04283461) of 105 healthy participants provided the basis for
Moderna’s investigational new drug appl...
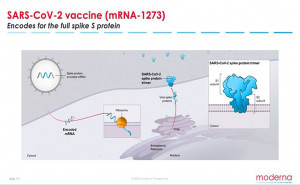
Moderna’s mRNA vaccine technology
offers potential advantages in efficacy, speed of development, and production
scalability and reliability.
mRNA-based vaccines offer several
advantages, including:
Ability
to mimic many aspects of natural viral infections.
mRNA enters cells and is used to
produce viral antigen...
The vaccine, known as mRNA-1273,
was co-developed by the Cambridge, Massachusetts-based American biotechnology
company Moderna, Inc., and the National Institute of Allergy and Infectious
Diseases (NIAID), part of the National Institutes of Health. The trial, which
will be conducted at U.S. clinical research sites, is expecte...
Background: The Oxford Vaccine Group at the University of Oxford
are developing a new vaccine candidate for COVID-19, a chimpanzee adenovirus
vaccine vector called AZD1222 (previously ChAdOx1). The team has previously
developed a MERS vaccine.
In India, the candidate is being
jointly developed by the Serum Institute of India...
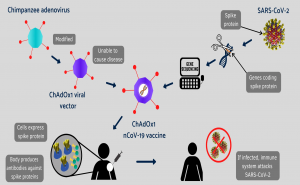
A diagram showing how the Oxford COVID-19 vaccine works. A chimpanzee
adenovirus is used in the ChAdOx1 viral vector, engineered to match the
SARS-CoV-2 spike protein.
The University of Oxford vaccine
is delivered via a chimpanzee virus, called the vaccine vector. The vector
contains the genetic code of t...
A vaccine in development by the
British-Swedish company AstraZeneca
and the University of Oxford is
based on a chimpanzee adenovirus called ChAdOx1.
Their Phase 1/2 trial revealed that the vaccine was safe, causing no severe
side effects. It raised antibodies against the coronavirus as well as other
immune defenses....
Background: CoronaVac (formerly PiCoVacc) is a formalin-inactivated
and alum-adjuvanted candidate vaccine. Results from animal studies showed
“partial or complete protection in macaques” exposed to SARS-CoV-2, according
to a paper published by researchers in the journal Science.
Study Design:
A Phase 1/2 trial enroll...
Chinese company Sinovac is
developing a vaccine based on inactivated Covid-19 particles. The vaccine has
shown a promising safety profile in the early stages of testing and is now
moving into Phase 3 trials in Brazil involving 9,000 volunteers. Already Phase
3 trials for Sinovac's CoronaVac began last week in Indonesia involving...
There is an assertion that the
Sinovac vaccine uses the "outdated" technology of inactivated whole viruses, and that
such "next generation" technologies as mRNA vaccines (NIH/Moderna) and live adenovirus vaccines into which Covid genes are introduced
(Oxford/Astra Zeneca) are preferable.
There is no scientific basis for
this...