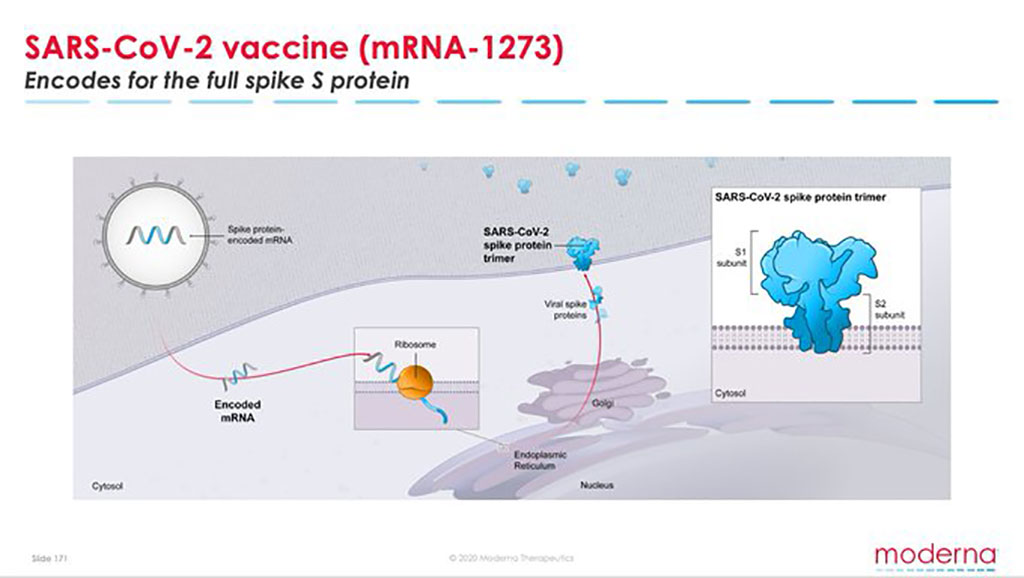
MODERNA (USA) AND NIH - mRNA-1273 CORONA VACCINE
The vaccine, known as mRNA-1273, was co-developed by the Cambridge, Massachusetts-based American biotechnology company Moderna, Inc., and the National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes of Health. The trial, which will be conducted at U.S. clinical research sites, is expected to enroll approximately 30,000 adult volunteers who do not have COVID-19.
The vaccine was tested in phase 1 trials on volunteers at the Kaiser Permanente Washington Health Research Institute in Seattle. Moderna has run phase 2 trials on participants of a wide range of ages and started phase 3 trials in July.
Moderna’s Potential Vaccine
(mRNA-1273) Against COVID-19
PHASE 3
In March, the company put the first Covid-19 vaccine into human trials, which yielded promising results, After carrying out a Phase 2 study they launched a Phase 3 trial on July 27. The final trial will enroll 30,000 healthy people at about 89 sites around the United States.
A Phase 3 clinical trial designed to evaluate if an investigational vaccine can prevent symptomatic coronavirus disease 2019 (COVID-19) in adults has begun.
The trial is designed to evaluate the safety of mRNA-1273 and to determine -
· if the vaccine can prevent symptomatic COVID-19 after two doses.
· As secondary goals, the trial also aims to study whether the vaccine can prevent severe COVID-19 or laboratory-confirmed SARS-CoV-2 infection with or without disease symptoms.
· The trial also seeks to answer if the vaccine can prevent death caused by COVID-19 and whether just one dose can prevent symptomatic COVID-19, among other objectives.
Trial volunteers will receive two intramuscular injections approximately 28 days apart. Participants will be randomly assigned 1:1 to receive either two 100 microgram (mcg) injections of mRNA-1273 or two shots of a saline placebo.
The trial is blinded, so the investigators and the participants will not know who is assigned to which group.
Pipeline:
There are currently nine prophylactic mRNA vaccines in our development pipeline, with seven Phase 1 studies currently underway.
Notes:
No mRNA vaccine has ever been approved for an infectious disease, and Moderna has never brought a product to market. But proponents of the vaccine say it could be easier to mass produce than traditional vaccines.
Source:
https://www.nih.gov/news-events/news-releases/phase-3-clinical-trial-investigational-vaccine-covid-19-begins
https://www.modernatx.com/pipeline/modernas-mrna-clinical-trials-cmv-mma-zika-several-types-cancer-and-other-diseases




Comments
You must login to write comment