Coronavirus-vaccine-updates
It’s a two-shot vaccine, so
what happens if people miss their second shot? Is a single shot still
protective?
The Pfizer-BioNTech vaccine requires two injections, given 21 days apart,
to prime the immune system well enough to fight off the coronavirus.
Two shots are needed, and the second shot is required to...
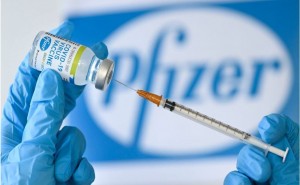
PFIZER AND BIONTECH VACCINE TO
COMBAT COVID-19
What is in the vaccine?
the Pfizer-BioNTech vaccine is based on the virus’s genetic instructions for
building the spike protein. The technology relies on a small piece of messenger
RNA (mRNA) from the coronavirus genome. It delivers a gene that codes for the
coronavirus'...
The German company BioNTech
partnered with Pfizer to develop and test a coronavirus vaccine known as
BNT162b2. The mRNA technology, short for messenger RNA vaccines like Pfizer's
are often referred to as the vaccines of the future, since the technology is
fairly new.
·
The active ingredient is messenger RN...
As the Pfizer-BioNTech COVID-19 vaccine is being evaluated by the U.S.
Food and Drug Administration (FDA) and the Moderna vaccine is soon to
follow, here’s a look at several of the top COVID-19 vaccine candidates
and where they stand as of today.
Pfizer and BioNTech Announce Vaccine Candidate Against COVID-19 Achieved Success in First Interim Analysis from Phase 3 StudyVaccine
candidate was found to be more than 90% effective in preventing
COVID-19 in participants without evidence of prior SARS-CoV-2 infection
in the first interim efficacy analysisAnalysis evaluated 94 confirmed cases...
Bangladesh has entered into a deal with the Serum Institute of India (SII) to acquire 30 million doses of a potential vaccine being developed by AstraZeneca for Covid-19.SII and Bangladesh’s drugmaker Beximco Pharma signed a Memorandum of Understanding (MoU) for priority delivery of the vaccine doses.Under the deal, Beximco will purchase five milli...
Details
Background: The Gamaleya Research
Institute in Russia is testing their non-replicating viral vector COVID-19
vaccine candidate, Sputnik V (formerly Gam-COVID-Vac), in two Phase 1/2 trials.
The trials recruited about 38 participants each to receive the vaccine
candidate (NCT04436471) (NCT04437875).
Outcomes...
Pharmaceutical company AstraZeneca and
Oxford University have resumed clinical trials of their COVID-19 vaccine
candidate in the United Kingdom after a brief global pause in testing.
AstraZeneca
put a hold on its COVID-19 clinical trials worldwide this week while it
investigated an adverse reaction in...
Oxford
researchers halt vaccine trial while adverse reaction is investigated
One
of the leading covid-19 vaccine candidate trials has been voluntarily paused as
part of a standard review process triggered by a “single event of an
unexplained illness that occurred in the UK phase III trial.”
The...
Beximco Pharmaceuticals, one of
the leading drug makers of the country, announced today it will invest with
Serum Institute of India (SII) to ensure Bangladesh receives Covid-19 vaccine
on a priority basis, once approved by the regulator.
In a press statement, Beximco
Pharmaceuticals said it will make a financial contribution...
The Covid-19 vaccine candidates by University of Oxford-AstraZeneca, Moderna Inc, Pfizer Inc-BioNTech and Chinese firm Sinovac & Sinopharm are all undergoing Phase III trials at present. Oxford-AstraZeneca coronavirus vaccine price:Adar Poonawalla, CEO of Serum Institute of India, said the cost of the vaccine is estimate...
The government has permitted icddr,b to run the Phase-III trial of a coronavirus vaccine developed by Chinese biopharmaceutical company Sinovac in Bangladesh.The Bangladesh Medical Research Council (BMRC) approved the vaccine in the middle of July more than a month after the icddr,b, local partner of Sinovac, submitted an application to...