Coronavirus-vaccine-updates
Novavax COVID-19 Vaccine has been
shown to be 89% effective in large-scale UK trials.
First to Demonstrate Clinical
Efficacy Against COVID-19 and Both UK and South Africa Variants.
The AstraZeneca-Oxford vaccine is
a recombinant adenoviral vector vaccine. Recombinant vaccines use a small piece
of genetic material from a pathogen, like SARS-CoV-2, to trigger an immune
response. A specific piece of the virus can be targeted, and recombinant
vaccines are generally safe to use in a large population of people—even those
with...
The ChAdOx1 nCoV-19 vaccine was
developed by the University of Oxford and AstraZeneca and manufactured in Serum
Institute of India.
The vaccine works by delivering
the genetic code of the SARS-CoV-2 spike protein to the body’s cells, similarly
to the BNT162b2 vaccine. Once inside the body, the spike protein is produced,
causing the immune...
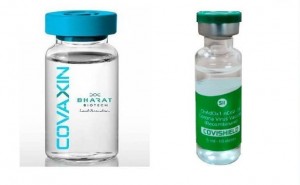
COVAXIN, India's indigenous
COVID-19 vaccine by Bharat Biotech is developed in collaboration with the
Indian Council of Medical Research (ICMR) - National Institute of Virology
(NIV). The indigenous, inactivated vaccine is developed and manufactured in
Bharat Biotech's BSL-3 (Bio-Safety Level 3) high containment facility.
The Drugs Controlle...
India has formally approved the
emergency use of two coronavirus vaccines.
The Drugs Controller General of
India (DCGI) granted its first emergency conditional approvals Jan. 3 for a
pair of COVID-19 vaccines, including Covishield, developed abroad by Astrazeneca plc
and Oxford University and manufactured by the Pune-based S...
The World Health Organization has
granted its first emergency use validation to BioNTech-Pfizer's COVID-19
vaccine.
UN health agency says emergency use
listing ‘opens the door’ for countries to expedite their vaccine approval
processes.
The World Health Organization has
listed Pfizer-BioNTech’s COVID-19 vaccine for emergency use, a crit...
The Oxford University
AstraZeneca coronavirus vaccine has been approved for use in the UK.
The Oxford University/AstraZeneca vaccine has been approved
by the UK medicines regulator, opening up the possibility of rapidly scaling up
vaccination against Covid-19 within days.
The Medicines and Healthcare products Regulatory Authority
(MHRA) has approved the 2 doses vaccine for use in the UK. Because it needs
only norma...
A two-dose regimen of Pfizer vaccine conferred 95% protection against Covid-19 in persons 16 years of age or older. Safety over a median of 2 months was similar to that of other viral vaccines.
The U.S. Food and Drug
Administration issued an emergency use authorization (EUA) for the second vaccine
— this one developed by Moderna for the prevention of coronavirus disease
2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2
(SARS-CoV-2).
Authorized Use
For the prevention of 2019
coronavir...
The basis of upcoming Pfizer and Moderna coronavirus vaccines. How it works?