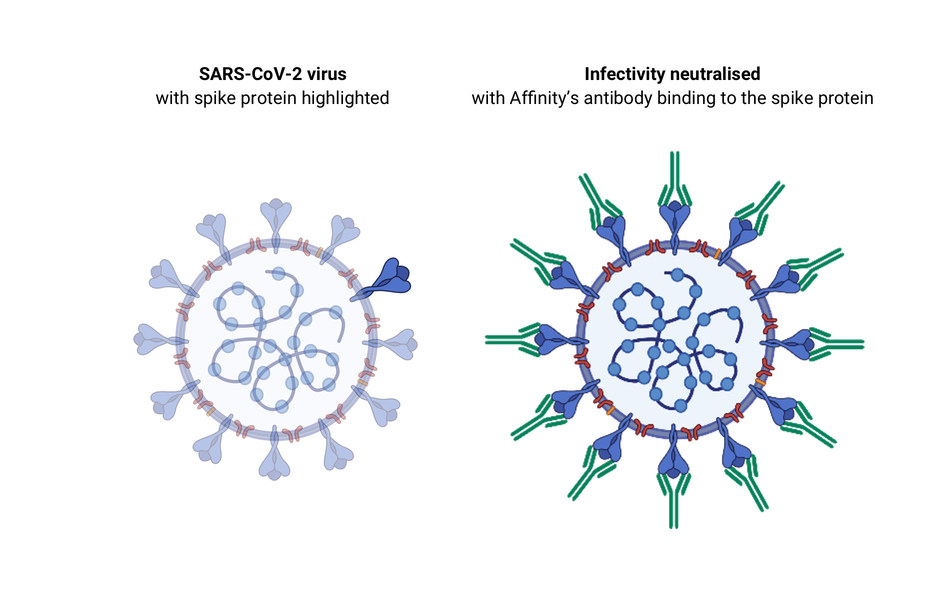
Monoclonal Antibody for Treatment of COVID-19 (P-2)
In a clinical trial of patients with COVID-19, monoclonal antibody were shown to reduce COVID-19-related hospitalization or emergency room visits in patients at high risk for disease progression within 28 days after treatment when compared to placebo. The safety and effectiveness of this investigational therapy for use in the treatment of COVID-19 continues to be evaluated.
Doctors Liked to Read More
Under the terms of the EUAs, mAbs may be used for the treatment of mild to moderate COVID-19 in adults and pediatric patients who meet all of the following:
· Have a positive test for SARS-CoV-2 (RT-PCR or antigen).
· Are within 10 days of the start of their symptoms.
· Are at least 12 years of age or older and weigh at least 40 kilograms (88 pounds).
· Are at high risk for progressing to severe COVID-19 and/or hospitalization.
High risk for progressing to severe COVID-19 and/or hospitalization is defined as patients who meet at least one of the following criteria:
· Have a body mass index (BMI) greater than 35.
· Have chronic kidney disease.
· Have diabetes.
· Have immunosuppressive disease.
· Are currently receiving immunosuppressive treatment.
· Are 65 years of age or older.
· Are 55 years of age or older AND have one or more of the following:
o Cardiovascular disease.
o Hypertension.
o Chronic obstructive pulmonary disease/other chronic respiratory disease.
· Are 12-17 years of age AND have one or more of the following:
o Body mass index greater than 85th percentile for their age and gender
o Sickle cell disease.
o Congenital or acquired heart disease.
o Neurodevelopmental disorders, for example, cerebral palsy.
o A medical-related technological dependence; for example, tracheostomy, gastrostomy, or positive pressure ventilation (not related to COVID-19).
o Asthma, reactive airway, or other chronic respiratory disease that requires daily medication for control.
MAbs should not be used for patients who are hospitalized due to COVID-19; who require oxygen therapy due to COVID-19; or who require an increase in their baseline oxygen needs due to COVID-19, in those on chronic oxygen therapy due to underlying non-COVID-19 related comorbidity. Treatment with bamlanivimab has not been shown to be of benefit in these groups, and may even result in worse clinical outcomes.
Bamlanivimab is not authorized for patients who are hospitalized due to COVID-19 or require oxygen therapy due to COVID-19. A benefit of bamlanivimab treatment has not been shown in patients hospitalized due to COVID-19. Monoclonal antibodies, such as bamlanivimab, may be associated with worse clinical outcomes when administered to hospitalized patients with COVID-19 requiring high flow oxygen or mechanical ventilation.
Unfortunately, mAbs have not been sufficiently studied to make a recommendation for using it while pregnant. Fact sheets for bamlanivimab and casirivimab/indevimab say that they “should only be used during pregnancy if the potential benefit outweighs the potential risk for the mother and the fetus.”
Pregnant women need to talk with their doctor or other health care provider.
Similarly, there is no available data on the presence of mAbs in human or animal milk, the effects on the breastfed infant, the effects on milk production, or on possible health effects from breast milk. Women who are breastfeeding need to discuss the issue with their doctor or other health care provider.
In clinical trials of bamlanivimab and casirivimab/indevimab involving almost 3,000 people, two anaphylactic reactions (a severe allergic reaction) and five serious-infusion-related reactions have been reported. All reactions were treated and resolved.
For bamlanivimab, the most commonly reported side effects (in 2% to 4% of people) were nausea, diarrhea, dizziness, headache, itchiness, and vomiting. For casirivimab/indevimab, the most commonly reported side effects (0.8-1.6% of people) were nausea and vomiting, hyperglycemia, and pneumonia. Intestinal obstruction occurred in 1 patient receiving the highest dose (8,000mg) of casirivimab/indevimab; however, this was not felt to be related to the drug and this does not been authorized for use.
In the BLAZE-1 trial, nausea occurred in 3.9%, dizziness in 3.2%, and mild infusion reactions in 2.3% of antibody recipients, compared to 3.5%, 2.1%, and 1.4%, respectively, of placebo recipients. According to the FDA’s fact sheet for the EUA, one anaphylactic reaction and one serious infusion-related reaction were reported with infusion of bamlanivimab in ongoing, blinded trials.
Clinical studies evaluating the safety of mAbs are ongoing, so it is possible all of the risks are not known at this time. Because it is an antibody treatment, it is possible that bamlanivimab could interfere with your body’s own immune response to future infections with SARS-CoV-2 or your immune response to a vaccine for COVID-19.
https://www.health.state.mn.us/diseases/coronavirus/hcp/bamfaq.html#where1
https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-monoclonal-antibody-treatment-covid-19
https://jamanetwork.com/journals/jama/fullarticle/2774326
https://www.lilly.com/news/media/media-kits/bamlanivimab-covid19




Comments
You must login to write comment