Herd Immunity
More good news! In addition to last week’s announcement of very high (90%) COVID-19 vaccine efficacy emerging from the first interim analysis of a phase 3 trial [1], we received word that a second vaccine had similar initial efficacy at 94.5%.[2] Both of these candidates use an mRNA platform, a novel approach to human vaccines. This consistent 90%-plus efficacy for mRNA vaccines is significantly higher than my wildest dreams, provides hope for controlling the pandemic, and brings the concept of herd immunity back onto center stage.
Herd immunity refers to the “protection of susceptible individuals against an infection when a sufficiently large proportion of immune individuals exists in a population.”[2] Basically, this means that the likelihood of an infected individual coming into contact with a susceptible individual is reduced to below the level needed to sustain a chain of transmission. An outbreak slows, a pandemic ends.
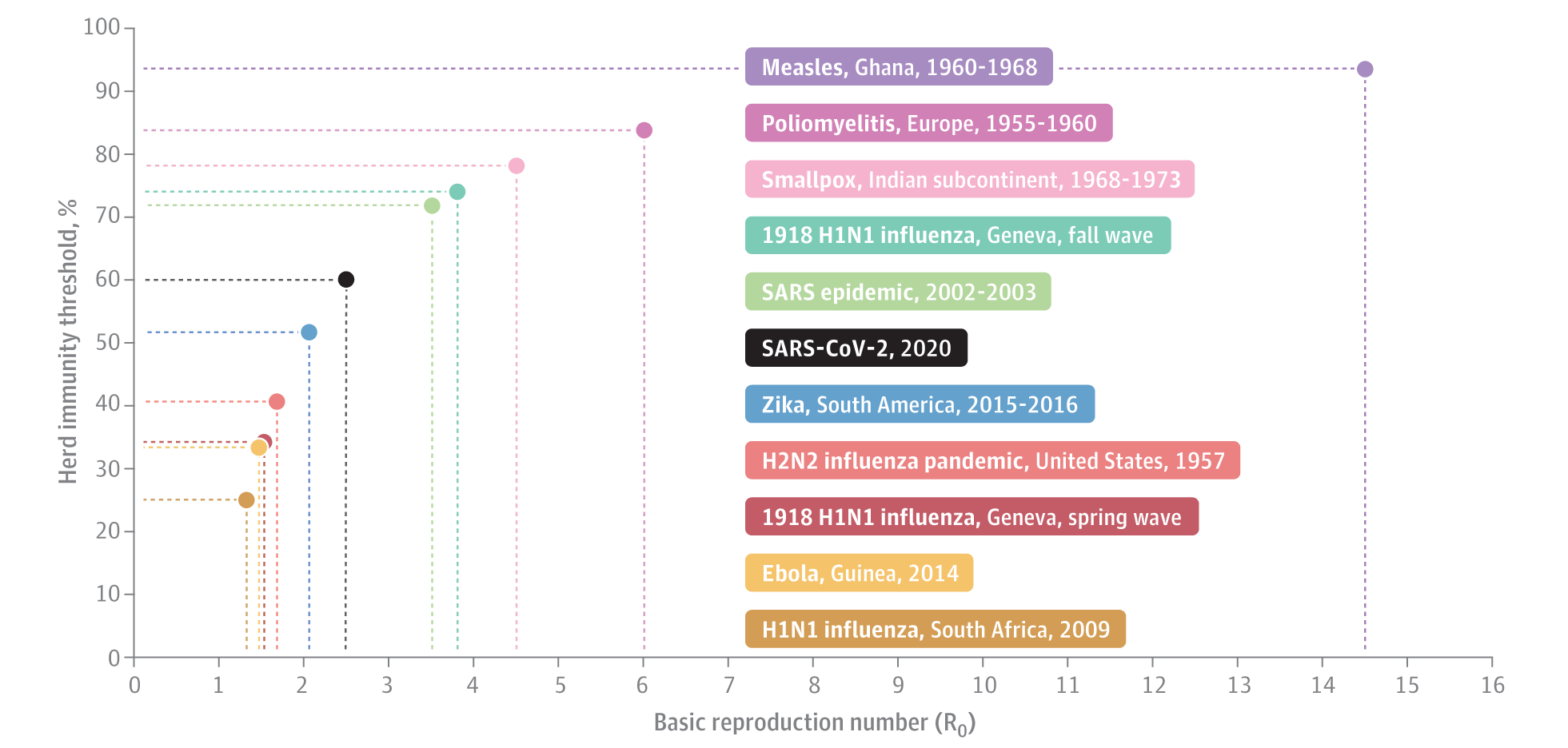
A succinct review of herd immunity is available and helps in the basic understanding of the interplay between infectivity and the requisite herd immunity threshold.[3] For highly transmissible viruses, like measles, very high immunity is needed to prevent outbreaks. For SARS-CoV-2, with a basic reproduction number (Ro) between 2 and 3 (i.e., 2—3 new cases generated per existing cases in a population of susceptible individuals), the estimated herd immunity threshold is around 60%.
We can attain the threshold through infection; the consequences in terms of morbidity and mortality, however, are untenable. We can also arrive at this magical place through vaccination. And this is where news of preliminary estimates of high efficacy are crucial. We will need high vaccine efficacy and high COVID-19 vaccine coverage rates to carry us over the heard immunity threshold. The vaccine efficacy part is on the scientists and manufacturers creating the vaccines. Vaccine coverage is on us.
Primary care clinicians and other healthcare workers will be among the first to receive vaccines for COVID-19. By our example, we hope to lead our patients, families and friends to acceptance. Better understanding of concepts like herd immunity is important across the whole of our population. Accordingly, patient information, such as the JAMA Patient Page on herd immunity, can be a worthwhile tool in your upcoming vaccine discussions.[4]
Source:
- Pfizer. Pfizer and Biontech announce vaccine candidate against COVID-19 achieved success in first interim analysis from phase 3 study [news release]. Accessed 11/11/2020.
- Moderna. Moderna’s COVID-19 Vaccine Candidate Meets its Primary Efficacy Endpoint in the First Interim Analysis of the Phase 3 COVE Study [News Release]. Accessed 11/17/2020.
- Omer SB, Yildirim I, Forman HP. Herd Immunity and Implications for SARS-CoV-2 Control. JAMA. Published online October 19, 2020. doi:10.1001/jama.2020.20892
- Desai AN, Majumder MS. What Is Herd Immunity? JAMA. Published online October 19, 2020. doi:10.1001/jama.2020.20895




Comments
You must login to write comment